<< View SCS Blog
The Treatment of Atrial Fibrillation with Parylene-Protected Pulsed Field Ablation Devices

The human heart beats somewhere around 100,000 times a day and generally does so with amazing reliability. The workings of the cardiac conduction system, the special electrical system of the heart that causes its various portions to contract in a top-to-bottom rhythm, enable the heart to pump blood throughout the entire body1.
For each heartbeat, the four chambers of the heart are activated; the upper two chambers called atria, and the lower two chambers called ventricles. The heartbeat sequence begins when the upper right atrium receives an electrical signal that quickly travels into the left atrium. This causes both atria to contract, pumping blood into the right and left ventricles. The signal then flows down to cells between the atria and ventricles, where it slows down just long enough for the ventricles to fill with blood. Then, the next signal fires, causing the ventricles to quickly contract and pump blood out of the heart to the rest of the body. At that same time, the atria are relaxing and subsequently filling with blood in preparation for the next contraction of the atria. The electrical cycle begins all over again for the next heartbeat, all in just under a second’s time.
Changes in heart tissue can create conduction disorders within the heart. These changes can either cause improper generation of the electrical signals or create roadblocks for those signals to properly travel through the heart, resulting in an irregular heartbeat, which is also known as arrhythmia. Atrial fibrillation, or Afib, is the most common type of arrhythmia and can result in elevated heartbeat, palpitations, dizziness, low blood pressure, extreme fatigue, blood clots or cardiac arrest. It is estimated that between the years 2010 and 2019, the global prevalence of Afib had risen from 33.5 million to 59 million people2.
Treatments for Afib often begin with the use of medication, including clot-preventing blood thinners, and can also include the insertion of a pacemaker or the use of a newer treatment called pulsed field ablation (PFA). PFA uses high amplitude pulsed electrical fields to create scarring on targeted heart tissue that helps break up or insulate the errant electrical signals that cause irregular heartbeats. This is accomplished by feeding the PFA device through a catheter to the heart and delivering the pulsed energy by direct contact to the targeted heart tissue. There are currently two FDA-approved PFA devices in the US market: Boston Scientific’s FARAPULSE™ PFA System and Medtronic’s PulseSelect™ PFA System.
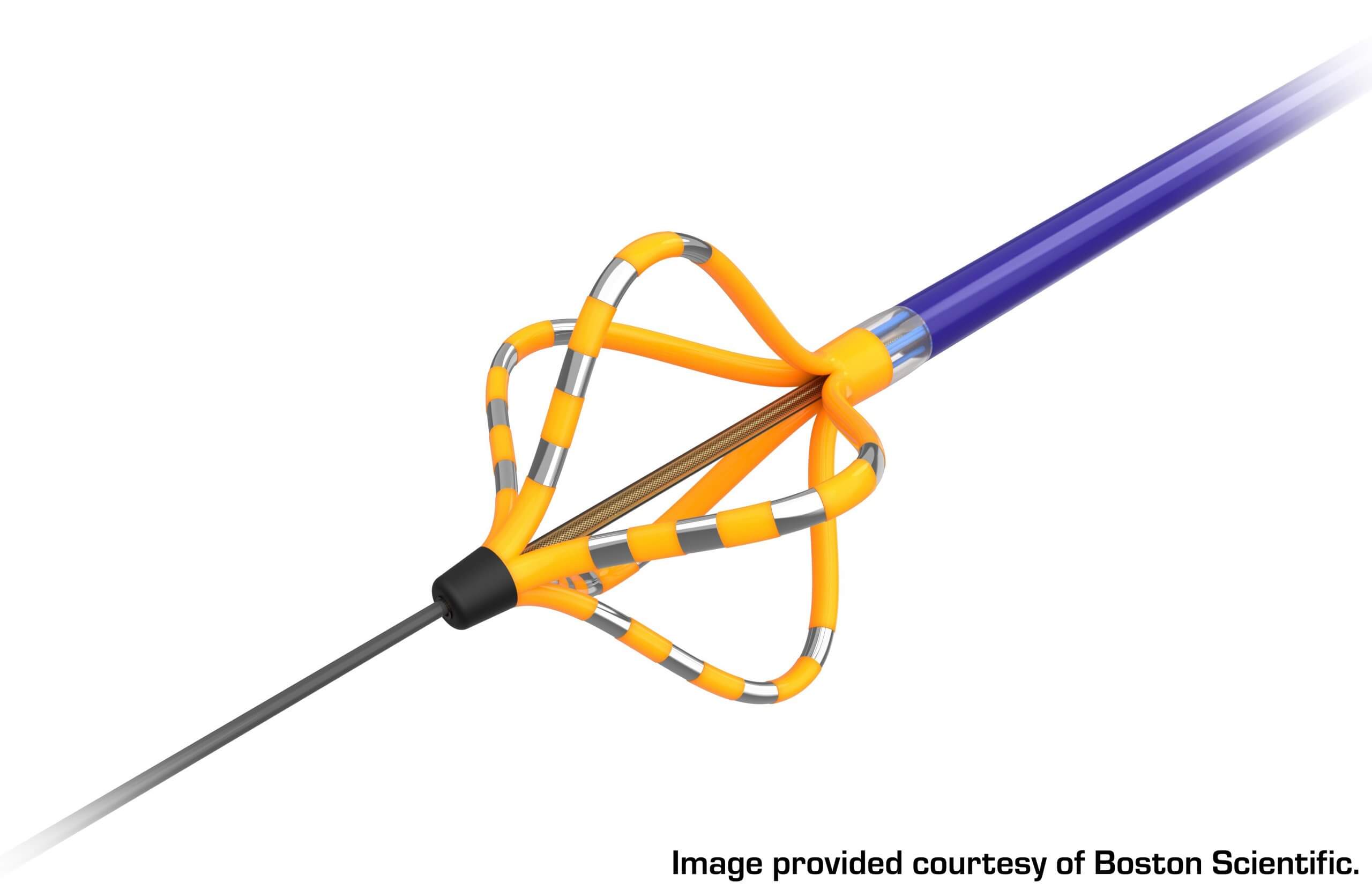
An example of one of these devices can be seen in Figure 2. The FARAWAVE™, which is the ablation catheter of the FARAPULSE System, has a basket-type configuration with silver-banded areas. The silver bands are the electrodes, or energy delivery locations, that are activated to send pulses to the tissue during the procedure. The yellow portions of the device are areas that are electrically insulated, allowing for the selective delivery of the pulsed electrical fields to the targeted tissue.
Manufacturers have limited options for insulation materials that meet today’s extensive device criteria as designers must take a variety of considerations into account, including insulation characteristics, biocompatibility, lubricity, environmental compliance and repeatability. Additionally, most catheter-type devices are subject to tight dimensional and tolerance requirements, making the thickness of the insulative coating a critical part of the design. While higher film thickness materials can be accommodated within the device design, variation in the uniformity of thicker coatings can become problematic if excessive.
Biocompatible and biostable, Parylene conformal coatings provide insulation and lubricity to increase the reliability of various devices and components. Parylenes are applied as ultra-thin and pinhole-free uniform layers that provide a thin-film insulator to accommodate for other components in the device design. In addition, Parylenes provide superior electrical insulation per unit thickness and a natural, dry lubricious surface. These are ideal characteristics for catheter-based procedural technologies such as PFA and, as a result, can improve performance and reliability.
Parylenes are utilized on approved medical devices around the world – from energized surgical technologies to long-term implants. The coatings are widely recognized for their biocompatibility and performance characteristics, and they play a crucial role in protecting current and next-generation medical device platforms.
For more information on how Parylene coatings can protect critical medical device applications, contact SCS online or call +1.317.244.1200.
References
- U.S. Department of Health and Human Services. (n.d.). How The Heart Works. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/heart
- Atrial fibrillation: Epidemiology, screening and Digital Health. (2024, February). https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00205-3/fulltext
*FARAPULSE and FARAWAVE are registered trademarks of Farapulse, Inc. PulseSelect is a registered trademark of Medtronic Inc.
Global Coverage Issue 98, Spring 2024